Polyure-Pain: Polyurethane Foams in Collections
- Bill Mastandrea
- Nov 20, 2020
- 10 min read
Plastics: A Quick Overview
Most people have this perception of conservation as a person sitting in a lab or a workshop carefully preserving an Old Masters' oil painting, a Roman sculpture, or an ornate piece of jewelry from ancient China. The reality is that not all of the objects in collections are ancient. People across the entirety of time have been creating new objects with new materials, and they matter just as much to the conservator as the "old stuff". For example, modern plastics are a HUGE area of research and developing understanding within the field of conservation. For example, I’ve spent the entirety of this week attending the virtual ‘Plastics in Peril’ conservation conference, listening to the incredible new research and techniques conservators and conservation scientists are developing.
Plastics are defined as a [partially or fully] synthetic material made from a wide range of organic polymers (Merriam-Webster) which can be molded or formed by heat and pressure (Science Museum). Cellulosic plastics were popular in the early 20th century, often used to mimic the appearance of rare and exotic tortoiseshell (see Figure 1). The Post-WW2 era saw the commercial availability of a massive range of new plastic materials: everything from 'Tupperware Everywhere' in the kitchen (polyethylene plastic) (see Figure 2), to reinforced reaction injection-molded (RRIM) components - such as the Enduraflex door panels (polyurethane hard plastic) used for the 1984 Pontiac Fiero (see Figure 3) - to flexible foams for cushioning and insulation used in the construction and automotive industries.
Figure 1: (A) Left image showing a tortoiseshell comb c. 1850 from the Horniman Museum Collection (33.131); (B) Right image showing a cellulose nitrate (a semi-synthetic plastic) comb c. 1900s from the Science Museum Collection (1980-676/92).

Figure 2: A vintage advertisement for polyethylene plastic Tupperware brand food-storage containers c. 1950s-60s (Image Source)

Figure 3: A vintage advertisement for the 1984 Pontiac Fiero, the first vehicle produced with all plastic body panels via reinforced reaction injection molded polyurethane. "Fiero's Innovative Enduraflex panels will never rust, the front fenders, door panels, and lower quarter panels "give" on minor impacts, and the other panels resist minor dents and dings." (Image Source: Hemmings).
Polyurethane is in Museum Collections?!
As part of my internship at the Horniman Museum and Gardens I was tasked with investigating Polyurethane (PURs), specifically foams, in order to recommend proper treatment and storage options for these really unstable plastics - which I will be going into more detail below.

Now, we've all seen PUR foams get yellow, crusty, and powder into nothing at home. "But Bill," I can hear you all saying, "There's polyurethane in museums?!" Yes! SO MUCH OF IT! So, get ready for a quick lesson on how pervasive PURs actually are and how quickly they become horrible and gross!
Polyurethanes were invented and patented in 1937 by Dr. Otto Bayer for the chemical company IG Farben (POPART 2012). The initial applications for PURs were in the form of fibers and foams used in aircraft in World War II, while PUR only became commercially available post-war in 1954 (POPART 2012).
“There are literally hundreds of different types of polyurethanes and each is made in a slightly different way…” resulting in a multiplicity of uses in commercial industry (Polyurethanes.org, 2020). Various forms include:
Thermoset plastics – as flexible and cross-linked foams (injection-molded, sprayed, or rigid slabstocks) used as insulation;
PUR-based rubbers – used as gaskets, seals, molded objects, floor coverings, and wheels;
Synthetic fibers – used for materials in the fashion, automotive, and art fields such as Spandex and Lycra;
Thermoplastics – used for imitation leathers and textile coatings; as well as
Adhesives and Varnishes
(Polyurethanes.org, 2020; Wikipedia 2020c; POPART 2012; Sá 2018).
Different PUR formulations alter the plastics properties changing the flexibility, rigidity, thermal stability and surface texture of the material. Their multiplicity of uses has meant that PURs have found their way into all manner of material used in the modern and contemporary art world and in what are now considered historic objects: from automotive and aviation history, to fashion, folk art, and build heritage.
Degradation of Polyurethanes
There are two major categories of PURs: polyester-based PURs and polyether-based PURs. The two forms differ in the materials used for their production, as well as their susceptibility to types degradation. Overall, PURs are susceptible to degradation via oxidation, photo-oxidation, and hydrolysis. The form these PURs take – whether foams, hard plastics, or rubbers – determines their susceptibility. The primary risk factors for PUR materials are light, heat, and moisture content (Balcar et al 2012; POPART 2012). Table 1 below, adapted from Williams (2002), shows the observable results of PUR degradation.

Table 1: Table of selected agents of deterioration on Polyurethane (Adapted from Williams 2002 and Rychlý and Rychlá 2012).
Ether-based PURs degrade primarily via oxidation and photo-oxidation whereas Ester-based PURs degrade primarily via hydrolysis, but are also susceptible to oxidation and photo-oxidation (POPART 2012). Oxidation and photo-oxidation degradation reactions of PURs will happen on their own once triggered, even in pure darkness (Williams 2002). Foams are especially susceptible, given their squishy foam structure which allows air to permeate the interior of the foam.
Oxidation reactions in PURs cause chain scissioning: the breaking apart of the larger polymer network into smaller chains. This chemical deterioration results in the characteristic physical changes we all know: powdering and disintegration (POPART 2012; Chaumat et al 2012). Because of the aggressive nature of PUR deterioration, PUR materials only have an expected lifespan of 20-50 years under 'normal' conditions – usually less for foams because of their squishy, porous structure (Sá 2018; Chaumat et al 2012). PUR degradation not only shortens the lifespan of the foams themselves, but the degradation process releases nasty organic acids which can damage neighboring materials – especially metals – promoting their corrosion (Sá 2018, 34-35; Rychlý and Rychlá 2012; Williams 2002).
What to Do, What to Do?

"But Bill," you surely must be saying, "If these foams deteriorate rapidly and harm themselves and other materials as they die, how do you store them and keep them alive?" Unfortunately, I don't have all the answers, but I'll share a bit about what options there are based on the research I found. Fear not, concerned citizen!
Most interventive treatments for PUR foams consist of consolidation or impregnation treatments, where a material is coated, sprayed, or allowed to permeate the foam, protecting it from light and contact with airborne moisture. Recent efforts, such as the European POPART Project (Preservation Of Plastic ARTefacts in museum collections) has dedicated time and resources to the scientific investigation of the materiality and degradation of industrial plastics which have seen use commercially and in art for the past 100 years.
As part of this project, six consolidant candidates (including acrylic materials, oils, and a material class called silanes, (see Tables 2 and 3) were studied for their efficacy in the stabilization and preservation of ester- and ether-based PUR foams. The most effective of which were alkoxysilanes and amino-alkylalkoxysilanes. These chemical compounds can be dissolved in organosilicon-based materials called siloxanes, creating a highly permeable liquid. These consolidants can effectively penetrate into foams and other materials, but unfortunately have the potential to be exceedingly harmful for humans and the environment. These concerns, alongside institutional issues around staff, time, and resources means that conservators are not always able to utilize them.
However, a more accessible long-term preservation option for PUR materials and PUR-containing objects is safe and suitable storage!
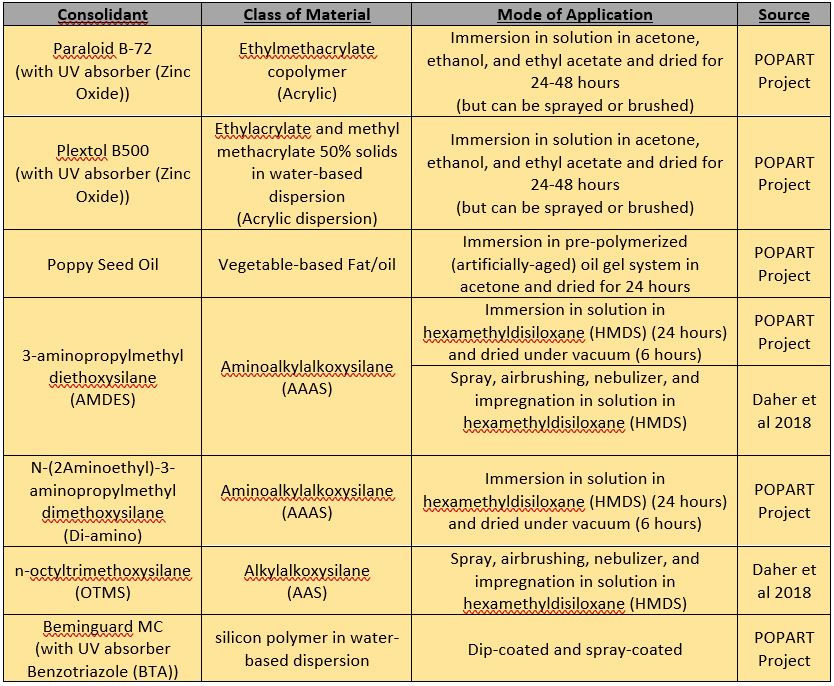
Table 2: Summary of recently tested consolidants in the consolidation of ester- and ether-based PUR foams (Chaumat et al 2012; Daher et al 2018).

Table 2: Summary of results of the recently tested consolidants in the consolidation of ester- and ether-based PUR foams (Chaumat et al 2012; Daher et al 2018).
Storing Polyurethane: A Self-Destructing Foam Beast
Three Cypriot carnival costumes made by Toula Sykopetritis for the 1989 Limassol carnival - a banana costume (2014.112.1-2), an octopus costume (2014.113), and a child's radish costume (2014.111) - in the Horniman Collection contain PUR foam and require a suitable solution to the inevitable and aggressive deterioration discussed above (see Figures 4-6 below). Given that the PUR foams in these costumes are either painted or attached to other material types, consolidation or impregnation treatment are not possible without endangering the neighboring materials. This leaves preventive storage measures as an accessible and suitable means of preservation.

Figure 4: The Cypriot banana costume covered in painted PUR bananas (2014.112.1-2) (Photo credit: Charlotte Ridley).

Figure 5: The Cypriot octopus costume with PUR suction cups on its tentacles (2014.113) (Photo credit: Charlotte Ridley).

Figure 6: The Cypriot radish costume, intended for a child, with textile-covered PUR foam radishes (2014.111) (Photo credit: Charlotte Ridley).
In general, as with other plastics, standard recommended storage conditions include:
A cold, dark, dry environment
Low-oxygen/anoxic (no-oxygen) conditions between 45-55% relative humidity (RH),
No exposure to UV light,
Good ventilation.
These conditions are generally acceptable for the costumes; from the synthetic textiles and metal components, to the polyvinyl acetate (PVAc) hot glues used in their manufacture. Michalski (2002) has remarked that materials which suffer from autocatalytic (self-destructive) deterioration can be assumed to last approximately twice as long for each 5°C drop in storage temperatures. It should be noted, however, that long-term low temperature storage is not practical or viable for every institution and is essentially inaccessible to smaller institutions or private collections.
For polyurethane specifically, there had been “…no systematic research for the definition of environmental conditions for the storage of this polymer” (Sá 2018, 37).
In an effort to fill the gap in understanding, Sá (2018) conducted storage tests of ester- and ether-based PUR foams under various conditions. Sample materials were subjected to 12 months of dark storage in one of four storage conditions:
Open-air at room temperature (~21°C);
Sealed in bags (combination Escal/aluminum barrier film) without oxygen removal at room temperature;
Sealed in bags without oxygen removal at low temperature (~12°C); and
Anoxic storage sealed in bags at room temperature.
A substantial find of the study revealed that, contrary to the accepted literature which warns that light damage for ether-based PUR specifically is most harmful,
“…it might be possible to attribute the discolouration to the presence and long-term availability of oxygen. Even though the anoxic storage was the only environment where this deterioration agent was lowered to <0.1%, the enclosed systems had oxygen contents around 20% or less, and after their consumption in oxidation reactions, this agent was no longer available for further deterioration” (Sá 2018, 196)
Take a look at the visual results from Sá's storage tests to see the massive difference in deterioration under each of the tested conditions!
Figure 7: Left - showing the results of the 12 month [t = time] dark storage of ether-based PURs; Right - showing the results of the 12 month [t = time] dark storage of ester-based PURs.
In both cases, the sealed enclosures limited exposure to oxygen. Since the oxidation of the PUR foams is dependent on the presence of oxygen, once all the available oxygen was used up in the degradation reactions, the degradation stopped. Therefore, for objects like the Cypriot carnival costumes, any of the enclosed storage options seem to be the most suitable options for long-term storage – with anoxic storage providing the ability to limit the greatest degree of oxidation prior to the depletion of oxygen in the storage environment. If the deterioration of the PUR can be limited, the resulting degradation products that would otherwise harm the neighboring materials can also be limited.
Considerations
While the findings from Sá (2018) are invaluable, there are a few more considerations that need to be made prior to storage. Enclosed storage of PUR or PUR-containing materials still places other material components at risk of deterioration – even if the oxidation reactions are unable to continue following the depletion of oxygen in the storage environment. This may, however, be a necessary step for the continued preservation of the entire object given that the potential deterioration or corrosion caused in other material components of an object are not themselves autocatalytic or caustic in nature.
As an example, wooden objects are known to have the ability to accept ‘imperfect’ RH and temperature conditions without risk of serious cracking, splitting, or warping if introduced gradually over time. Reintroduction to rapid fluctuations of these conditions will alter the wooden substrate unfavorably, resulting in cracks and warping. Similarly, it is considered that once acclimatized to the enclosed, low-oxygen storage space, rapid reintroduction to an oxygen-rich environment may have devastating consequences for PURs. It is therefore suggested that a means to slowly reintroduce oxygen into the stored object’s environment should the object need to be removed from storage is necessary to avoid potential catastrophic damage.
As with any enclosed object treatments, the regulation and monitoring of RH within the storage solution should not be allowed to be too high, promoting the production of condensation and therefore degradation by hydrolysis, or too low, putting organic components at risk of embrittlement and fracture. While costly, the use of a stabilizing silica gel, such as Prosorb, may be necessary to maintain favorable RH condition within the sealed enclosures over time.
This brief summary of recent research is certainly not exhaustive and there is undoubtedly a great deal more information available for those who are interested in the degradation processes, material identification and characterization, and conservation of modern plastics such as polyurethane. This brief summary is also not intended to be a definitive recommendation for the safe storage of PUR materials and PUR-containing materials. Instead, this is my attempt to share the experience of working as a conservator at an institution like the Horniman, where critical-thinking, creative problem-solving, and research are a necessary part of the job. While this is my first foray into the world of conservation of plastic materials, I found this area to be really interesting and I look forward to more opportunities to explore and investigate the really bizarre patterns of degradation of plastics! More blog posts to come as I keep up the hard work. Thanks everyone!
References:
Balcar, N., Lattuati-Derieux, A., and Vila, A., 2012. Appendix 3: Analysis of degradation products found during surveys of three French Collections. In: Lavédrine, B., Fournier, A., and Martin, G. (eds.), 2012. Preservation of plastic artefacts in museum collections. Paris: Comité Des Travaux Historiques et Scientifiques.
Barabant, G., 2012. Appendix 2: Degradation associated with some plastics found during surveys of three French collections. In: Lavédrine, B., Fournier, A., and Martin, G. (eds.), 2012. Preservation of plastic artefacts in museum collections. Paris: Comité Des Travaux Historiques et Scientifiques.
Chaumat, G., Tran, K., Matthijn Dekkers, J., Pellizzi, E., and Lattuati-Derieux, A., 2012. 4.3 On-going studies in consolidation of polyurethane (PUR) foams. In: Lavédrine, B., Fournier, A., and Martin, G. (eds.), 2012. Preservation of plastic artefacts in museum collections. Paris: Comité Des Travaux Historiques et Scientifiques.
Daher, C., Fabre-Francke, I., Balcar, N., Barabant, G., Cantin, S., Fichet, O., Chéradame, H., Lavédrine, B., and Lattuati-Derieux, A., 2018. Consolidation of degraded polyurethane foams by means of polysiloxane mixtures: polycondensation study and application treatment, Polymer Degradation and Stability, vol. 158, pp. 92-101,
Polyurethanes.org, 2020. What is polyurethane? [online.] Available at:
https://www.polyurethanes.org/en/what-is-it/#:~:text=Polyurethane%20is%20a%20plastic%20material,cushioning%20for%20furniture Accessed: 19 October 2020.
Preservation of Plastic ARTefacts in museum collections (POPART), 2012. Damage atlas: atlas of case studies presenting typical damages. [online.] Available at: http://popart-highlights.mnhn.fr/wp-content/uploads/3_Collection_survey/5_Damage_atlas/Damage_atlas.pdf
Accessed 19 October 2020.
Roff, W.j., Scott, J.R., and Pacitti, J., (eds.) 1971. Handbook of common polymers: fibres, films, plastics and rubbers. Cleveland: CRC Press and Butterworth & Co.
Rychlý J., and Rychlá, L., 2012. 3.1 Introduction. In: Lavédrine, B., Fournier, A., and Martin, G. (eds.), 2012. Preservation of plastic artefacts in museum collections. Paris: Comité Des Travaux Historiques et Scientifiques.
Sá, S.C.D.F., 2018. What does the future hold for polyurethane fashion and design?
Conservation studies regarding the 1960s and 1970s objects from the MUDE collection (PhD Dissertation). Ann Arbor: Proquest LLC.
Wikipedia, 2020a. Isocyanate. [online.] Available at: https://en.wikipedia.org/wiki/Isocyanate
Accessed: 19 October 2020.
Wikipedia, 2020b. Polyol. [online.] Available at: https://en.wikipedia.org/wiki/Polyol
Accessed: 19 October 2020.
Wikipedia, 2020c. Polyurethane. [online.] Available at: https://en.wikipedia.org/wiki/Polyurethane Accessed: 19 October 2020.
Williams, R. S., 2002. Care of Plastics: Malignant Plastics, WAAC Newsletter, vol. 24, no. 1 [online.] Available at: https://cool.culturalheritage.org/waac/wn/wn24/wn24-1/wn24-102.html Accessed: 19 October 2020.











Comments